

The diagnosis of internal parasites in companion animals continues to evolve. Efficient methods that allow clinicians to diagnose infections more quickly and implement treatment earlier have helped pets live longer, healthier lives. Because some internal parasites spread zoonotic disease, such advances also help protect owners.
PARASITE CLASSIFICATION & IDENTIFICATION
There are 2 major categories of internal parasites that affect both dogs and cats:
1. Protozoans (eg, Giardia, Toxoplasma, Tritrichomonas)
2. Helminths (eg, roundworms, hookworms, tapeworms).
Although most parasites are associated with a particular system of the body, due to life cycle differences, evidence of their presence may be seen in a variety of places (Table 1). For this reason, more than 1 method of testing may be needed to confirm parasitic presence. In addition, attention to detail with regard to health history, aseptic collection, and processing of relevant samples as well as clinical experience in the laboratory help determine diagnostic answers.
|
TABLE 1. Diagnostic Testing Organized by Body System & Target Parasites |
|
|
Diagnostic Test |
Parasite Classification |
|
GASTROINTESTINAL ANALYSIS |
|
|
Passive fecal flotation |
Helminth eggs |
|
Vomit flotation |
Nematode ova (helminth) |
|
Fecal sedimentation |
Helminth ova (especially trematode ova) |
|
Baermann technique |
Lungworm larvae in feces |
|
Fecal culture |
Difficult-to-identify eggs and cysts |
|
Stained fecal smear |
Protozoan oocysts and trophozoites |
|
BLOOD ANALYSIS |
|
|
Direct blood examination |
Heartworm microfilaria |
|
Modified Knott’s test |
|
|
Buffy coat method |
|
|
ELISA testing |
Heartworm antigens and antibodies |
|
Stained blood smear |
Blood protozoa |
|
URINE ANALYSIS |
|
|
Urine sedimentation |
Helminth ova |
GASTROINTESTINAL ANALYSIS
The gastrointestinal (GI) tract plays host to a variety of helminths and protozoan parasites. Because these parasites may mature and reproduce in different areas of the GI system, there is no single best method for ova, larvae, or adult identification.
Likewise, the method and choice of flotation solution that may be used to identify parasitic ova, oocysts, or larvae will differ based on specific gravity (SG) of the organism compared to that of the chosen flotation solution. If protozoan infection of the GI tract is suspected, different testing methods may be used to identify and differentiate between oocysts and trophozoites.
The choice of solution as well as method of analysis contributes to the success or failure of accurate analysis. Table 2 lists fecal flotation solutions and the parasites identified through their use.1
|
TABLE 2. Quick Guide to Solutions & Target Parasites1 |
|
|
SOLUTION |
IDENTIFIABLE PARASITES |
|
Sodium chloride*† |
Common helminths |
|
Sheather’s* |
Common helminths |
|
Sodium nitrate*† |
Common helminths |
|
Zinc sulfate* |
Common helminths (particularly Giardia) |
|
Magnesium sulfate*† |
Common helminths |
|
*Noneffective if flukes are suspected; use of fecal sedimentation is advised |
|
PARASITOLOGY PRIMER
Protozoa Life Stages
Cyst: Infectious form of many protozoan parasites during which they are encapsulated inside a protective wall; usually found in the feces
Oocyst: Encysted, highly resistant zygotic stage of some sporozoan parasites that may remain infective for extended periods of time
Trophozoite: Active, motile feeding stage of the flagellate protozoa as well as the postsporozoite state that is seen in some apicomplexan parasites (Figure 1)
Adult: Mature form of protozoan life capable of sexual or asexual reproduction
Helminth Life Stages
Ova: Scientific term for cells commonly referred to as “eggs”; singular is “ovum”
Larva: Independent, immature (ie, juvenile) worm that must undergo maturation in form and size to achieve adulthood
Adult: Mature helminth life form capable of reproduction
Helminth Types
Trematode: Of the class Trematoda, a parasite unique in its flat-bodied appearance (ie, liver fluke) and ability to parasitize certain organs, such as the liver (Figure 2)
Cestode: Of the class Cestoda, a parasite with a ribbon-like flattened body and specialized “hold-fast” organ of attachment at one end; commonly referred to as tapeworms (Figure 3)
Nematode: Of the class Nematoda, a parasite with an unsegmented and tubular, elongated body; commonly referred to as roundworms (Figure 4).
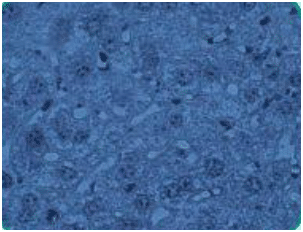
Figure 1. Trophozoites in liver tissue section
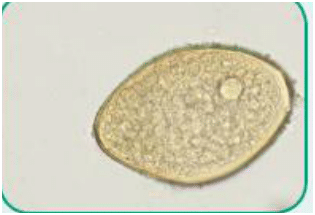
Figure 2. Ova of Fasciola hepatica, also known as common liver fluke (trematode)
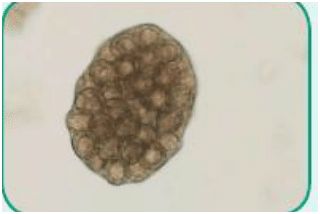
Figure 3. Ova of Taenia species, also known as tapeworms (cestode)
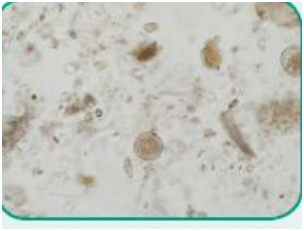
Figure 4. Ova of Toxocara cati, also known as feline roundworm (nematode)
Fecal Flotation
Fecal flotation remains the most common method to detect helminth eggs and protozoan cysts.
Flotation Solutions
Based on the SG differences of the various parts of a fecal sample (feces, ova, cysts, debris), the recovered eggs are lighter (ie, lower SG) than the flotation solution and will float to the surface. The heavier fecal matter (ie, higher SG) sinks rapidly.
Therefore, the flotation solution must have a higher SG than the parasite eggs or cysts. It is important to note that while water may often be a part of a fecal flotation solution, pure tap water alone cannot be used because the eggs and oocysts are heavier, sinking in the tap water and remaining unidentified.
There are several commonly used fecal flotation solutions that clinicians can quickly make for clinical use (Table 3).1 Choose solutions based on health history and expected findings (Table 2).1
|
TABLE 3. Fecal Flotation Solutions1,2 |
||
|
SOLUTION |
SPECIFIC GRAVITY |
FORMULATION |
|
Sodium chloride* |
1.2 |
Add table salt to boiling water until the salt no longer dissolves |
|
Sheather’s† |
1.2–1.25 |
454 g (1 lb) sugar + 355 mL water |
|
Sodium nitrate |
1.2–1.33 |
315 g sodium nitrate: 1 L water |
|
Zinc sulfate |
1.18 |
386 g zinc sulfate: 1 L water |
|
Magnesium sulfate |
1.32 |
350 g Epson salts: 1 L water |
|
*Sodium chloride solution, although widely used due to its low cost, is highly corrosive and will distort eggs |
||
Passive Fecal Flotation
Passive fecal flotation is conducted by mixing fecal matter and flotation solution in a small vial to form a slurry; the vial is then filled until a positive meniscus forms (Figure 5). A cover slip is placed over the meniscus and left undisturbed for 10 minutes, which allows the eggs/cysts to float to the top (Figure 6). The cover slip is then placed on a microscope slide and scanned at low power for signs of ova or larvae.2
Centrifugal Flotation
Centrifugal flotation “spins down” fecal debris, allowing the eggs/cysts to float to the solution’s surface. Slide preparation is based on the type of centrifuge used (fixed head versus swing head).
There are several centrifugal fecal flotation devices currently available that include a sieve to strain the mixture, which provides a cleaner sample for analysis; these devices include the Fecatector (butlerschein.com), Fecalyzer (vetoquinolusa.com), and Ovassay Plus (zoetis.com).

Figure 5. Positive meniscus

Figure 6. Cover slips placed over menisci for fecal flotation test
Passive versus Centrifugal Flotation
Numerous published studies have established that centrifugation is the preferred technique for parasite identification,3-6 and is, therefore, the technique recommended by most parasitologists. The centrifugal force allows a more rapid, efficient, and thorough separation of eggs/cysts and debris.
VOMIT FLOTATION
While not common, it is possible to identify some nematode ova by evalsuating vomit using the same methodology as for fecal flotation. Likewise, vomit may also be scrutinized under a microscope to locate parasites common to the stomach.
Vomit flotation is useful when parasites, such as Physaloptera species or Ollulanus tricuspis, are suspected in dogs and cats.2 Vomit flotation should also be included in the initial workup for companion animals that present with chronic vomiting.2
FECAL SEDIMENTATION
The fecal sedimentation test is used to detect ova that have a SG higher than commonly used flotation solutions and, therefore, do not readily float. This method may also be used for ova that will be distorted or destroyed in the presence of a super saturated salt solution.
The sedimentation test is most valuable for identifying fluke ova, which have a higher SG and are larger, denser, and heavier than other ova, and should be performed in suspected cases of infection.1 Also, because some nongastrointestinal inhabitants, such as Paragonimus kellicotti (lung flukes), may also shed ova in sputum or other internal fluids, microscopic examination should be considered.2
BAERMANN TECHNIQUE
The Baermann testing method (often referred to as the “Baermann technique”) involves the concentration of nematode larvae from a fecal sample. Although this technique is useful for identification of most nematodal larval forms, it is the gold standard method of testing for suspected cases of lungworm infection. Table 4 lists the most common lungworm species seen in domestic animals.7,8
|
TABLE 4. Common Lungworm Species Seen in Domestic Animals |
|
|
Lungworm Species |
Domestic Animal Host |
|
Aelurostrongylus abstrusus |
Cats |
|
Capillaria aerophila |
Cats |
|
Dictyocaulus arnfieldi |
Donkeys and horses |
|
Dictyocaulus eckert |
Deer |
|
Dictyocaulus viviparus |
Cattle |
|
Metastrongylus apri |
Pigs |
|
Muellerius capillaris |
Sheep and goats |
|
Oslerus osleri |
Dogs |
|
Protostrongylus rufescens |
Sheep and goats |
Successful larval collection relies on the fact that most nematode species lack the ability to swim against gravity. When lungworms are present in the lower respiratory tract, larvae within the airways move with sputum to the pharynx where they are swallowed and later shed in the feces. Thus, in the case of lungworms, no eggs are found in the feces, only larvae.7While there are several variations to the treatment of the sample in terms of staining and observation, the basic principle for the Baermann technique remains rooted in the simple apparatus required.
Step by Step: Baermann Technique
1. Break up fecal specimen and wrap loosely in cheesecloth.
2. Place specimen in tea strainer that is suspended over funnel.
3. Clamp rubber tube closed (tube is attached to end of funnel).
4. Pour lukewarm water into the funnel, covering cheesecloth packet.
5. Allow specimen to sit undisturbed overnight.
6. The next day, slowly relax clamp to collect a drop of fluid on a microscope slide. Add 1 drop of Lugol’s iodine to stain larvae and view (10×) with cover slip.
7. Alternatively: Release 1 to 2 drops of fluid into small test tube. Add 1 drop of Lugol’s iodine, tap mixture, place drop on slide and, with aid of cover slip, view at 10×.
Step by Step: Fecal Sedimentation Test2 (Figure 7)
1. Mix 2 g of feces with water in a cup (A); remove debris by straining mixture through gauze or cheesecloth into a centrifuge-safe tube (B and C).
2. Balance tube and centrifuge at 1500 rpm for 5 minutes (D).
3. Pour off liquid without disturbing the sediment.
4. Pipette a small amount from the top layer of sediment (E) onto a microscope slide; apply a cover slip for viewing. If sediment is too dense and visibility is obscured, dilute with a drop of water before examining.
Notes:
· If protozoan cysts are suspected, dilute sediment with Lugol’s iodine for easier viewing.
· If protozoan trophozoites are suspected, evalsuate a smear of the sediment prior to adding Lugol’s iodine, as iodine kills living trophozoites, impeding visible motility.

Figure 7-A

Figure 7-B
Figure 7-C

Figure 7-D
Figure 7-E
FECAL CULTURE
Culturing fecal material is a useful technique when eggs or cysts cannot be properly distinguished or identified. Incubation of the culture at room temperature encourages larval hatching and allows for easy identification.2
STAINED FECAL SMEAR
As a confirmatory method of identifying protozoan trophozoites, the fecal smear may be stained. The stain allows identification of oocysts and trophozoites by highlighting protozoan internal structures.
Since the iodine stain will kill trophozoites, it is prudent to examine the fecal smear for motile organisms prior to staining; the motility of trophozoites is what renders them easily identifiable. Nonmoving organisms may be difficult to identify within the stained smear.1,2
Step by Step: Stained Fecal Smear1,2
1. Mix a small amount of feces with saline on a microscope slide, creating a thin layer.
2. Using a cover slip, move any fecal material out of the way (to the side) before placing the cover slip on the smear.
3. Examine the slide using 10x and 40x (do not use oil immersion). Trophozoites are motile when unstained.
4. Stain the smear by applying Lugol’s iodine (add 1 drop to smear), which will stain the organism’s internal structures. The trophozoites will no longer be moving after iodine application.
BLOOD ANALYSIS
Heartworms
Diagnosis of heartworms is both simple and complex, and dominates veterinary parasitological research. A major focus of research involves identification of this parasite as early as possible postinfection in order to facilitate timely treatment.
Step by Step: Fecal Culture2
Nematode Larvae
1. Place 20 to 30 g of fresh feces in a glass jar. Break up feces and moisten with water; do not add enough water to make the solution soupy.
2. Store away from direct sunlight at room temperature for 7 days. Observe daily for condensation inside jar. If there is no condensation, mist contents lightly to maintain a moist environment.
3. On day 7, collect drops of moisture with small brush or inoculation loop and transfer them to microscope slide.
4. Stain the slide with iodine and kill larvae by passing slide over Bunsen burner. Killed larvae extend their bodies, making identification easier.
Coccidial Oocysts (Figure 8)
1. Mix 20 to 30 g of fresh feces with 60 mL of 2.5% potassium dichromate (A and B).
2. Pour mixture into petri dish (C), cover with lid, and incubate at room temperature for 3 to 5 days. Aerate each day by gently swirling contents with lid removed (D).
3. On day 5, use fecal sedimentation to evalsuate the sample.
4. Follow up by processing fecal sediment with centrifugal fecal flotation; then examine microscopically for oocysts.
Figure 8-A

Figure 8-B

Figure 8-C

Figure 8-D
Protozoan Parasites
Other parasites may infect various blood cells.
For instance, Hepatozoon is transmitted by ingestion of infected ticks, most notably Amblyomma maculatum and Rhipicephalus sanguineus. It should be noted that H americanum occurs only in North America and is carried by A maculatum. Hepatozoon presents on the surface of polymorphonuclear leukocytes as well as in skeletal muscle.9
Other protozoan parasites, such as Babesia canis, are transmitted through the bite of the tick Rhipicephalus sanguineus. B canis presents within canine erythrocytes.
HEARTWORM IDENTIFICATION
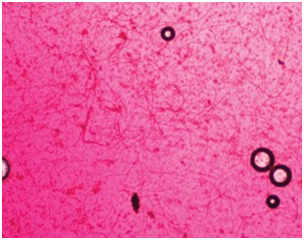
Figure 9. Dirofilaria immitus microfilariae identified by concentrated filtering technique with staining at 10x (above) and 40x (below)
The 3 manual methods routinely recognized for immediate detection of microfilariae are:
· Direct blood examination (examining a drop of blood on a slide)
· Modified Knott’s test (examination of centrifuged blood sediment)
· Examination via concentrated filter test (Figure 9).
The buffy coat method isolates motile microfilariae within the buffy coat layer, which is seen in centrifuged hematocrit tubes of whole blood, located between the red blood cell layer and plasma. This method, while considered fast, requires more prep time than the methods mentioned above. It should not be relied upon for definitive diagnosis because microfilaria may be difficult to see.
Use of enzyme-linked immunosorbent assay (ELISA) testing methods, such as the SNAP Heartworm Test or SNAP 4Dx Plus Test (idexx.com), are also useful for in-clinic detection of either antigen or antibody in patients with or without clinical signs of heartworm disease but no evidence of circulating microfilaria detected through manual examination of blood (due to lack of reproducing females at time of testing).
Testing for antigens produced by parasites remains the most widely used method of detection with regard to heartworm disease as it is quick and economically feasible. It should be noted that more than 1 method is often used to confirm results, whether negative or positive.
Step by Step: Buffy Coat Method9
1. Prepare a hematocrit tube using whole blood. Be sure to seal one end of the tube with sealant clay.
2. Process the tube in a microhematocrit centrifuge at 1500 rpm for 5 minutes.
3. Place centrifuged tube on microscope stage and, using 4x objective with low light, examine the area between the buffy coat and plasma for microfilariae movement.
4. If desired, break tube at the buffy coat layer and tap plasma onto a slide. Add a drop of methylene blue stain, a drop of physiological saline, and cover slip. Observe under 10x objective, with low to medium light, and scan for microfilariae.Figure 9. Dirofilaria immitus microfilariae identified by concentrated filtering technique with staining at 10× (A) and 40× (B).
Protozoa Identification in Blood
Diagnosis of unicellular parasites is easily accomplished in a clinical setting by examination of a stained blood smear. The best samples to evalsuate are those from animals with an acute infection. A more definitive diagnosis or confirmatory testing (such as in the case of Babesia) may be achieved through diagnostic serology as well as utilization of diagnostic laboratory services.
Staining may be accomplished using 1 of the following stains:
· Giemsa
· Quik-Dip Stain (mercedesmedical.com)
· Dip-Quick Stain (jorvet.com).
The gold standard for blood protozoan identification is Giemsa stain, but the quick-dip, 3-step systems also yield satisfactory results, of which several varieties are commonly used in clinical practice.9
Step by Step: Giemsa Staining of Blood Film2
1. Air dry a prepared blood film.
2. Fix in methanol for 5 minutes and air dry again.
3. Cover with Giemsa stain (1:20 Giemsa:distilled water ratio).
4. Allow to stand for 30 minutes undisturbed.
5. Gently rinse slide with tap water.
6. Air dry and view.
Note: Parasite cytoplasm will stain blue while the nucleus will stain pink/magenta.
ANALYSIS OF URINE
There are several parasites restricted to the urinary system, such as the giant kidney worm (Dioctophyma renale) and bladder worm (Pearsonema plica). The presence of these parasites is most easily confirmed by identifying the presence of ova in urine.
Ova may be identified by examining urine sediment samples collected through cystocentesis. To avoid contamination, cystocentesis is preferred over clean catch, voided specimens. Prior to testing, sample integrity should be guarded to avoid outside contamination, such as contact with insects, pollen, or other environmental debris.2,9
Step by Step: Urine Sedimentation2
1. Place 5 to 10 mL of urine in a conical tube; centrifuge at 1500 to 2000 rpm for 5 minutes.
2. Decant supernatant; examine the approximately 0.5 mL of sediment left in the tip of the tube.
3. Re-suspend by stirring with a wooden stick or pipette and transfer a drop to a microscope slide.
4. Add a cover slip and examine the slide for ova.
Note: I have had some success staining the sediment with Sedi-Stain (bd.com) prior to examination.
IN SUMMARY
· Identification of some parasites requires aseptically collected specimens.
· Specimen integrity must be preserved during collection, transportation, and testing.
· An up-to-date health history in conjunction with laboratory testing will allow the clinician to make a more accurate diagnosis.
· Laboratory testing should be conducted using established protocols in order to minimize human error. n
ELISA = enzyme-linked immunosorbent assay; GI = gastrointestinal; SG = specific gravity
