

Pressures on centralized, lab-based diagnostic services are expected to increase in the coming years with an aging population. The NHS responded to this emerging scenario in mid-2015 with a Personalized Medicine strategy that proposes earlier use of Point of Care (PoC) diagnostics and real-time monitoring of conditions to achieve the best outcomes.
Centralized, lab-based diagnostics will struggle to provide this desired future capability. In addition to the existing healthcare scenario, a number of additional scenarioses have emerged in recent years creating a requirement for a different type of diagnostic capability.
The first scenario is the development of antimicrobial resistance (AMR) that threatens to make the use of antibiotics ineffective. Broad spectrum antibiotics are often prescribed by clinicians to sick patients when the clinician lacks rapid actionable diagnostic results. Current diagnostic techniques that rely on traditional bacteria culturing methods can potentially take days to provide a result.
Epidemics are the second challenging diagnostic scenario. The 2014 outbreak of Ebola in West Africa, for instance—the largest outbreak of Ebola in history—highlighted a lack of practical, rapid, and consistent tests to confirm the disease. Current diagnostic methods are largely lab-based & used in a reactive manner.
There has been some limited success in developing tools to relocate diagnostic capability outside of the central lab, with the most prominent example being Cepheid’s GeneXpert, although this is not without issues as the system is reportedly too complex and expensive to be distributed at primary healthcare centers.
Analysis of these scenarioses identifies the following requirements for a new generation of PoC diagnostic systems:
Identifying these requirements is the first step in addressing emerging unmet needs in PoC diagnostics.
Limitations of Manufacturing Methods
Existing commercial diagnostic systems comprise multiple components fabricated using differing processes (e.g. injection moulding, micro-fabrication) that are then assembled while satisfying strict sterility and regulatory requirements for traceability.
These "traditional" manufacturing processes involve large capital investment to set up and maintain, which creates potential for a risk-averse R&D policy with new products constrained to manufacture via existing manufacturing infrastructure. Though this is understandable from a financial perspective, this situation can lead to iterative improvements in generations of products rather than allowing truly disruptive developments to be commercialized.
Recent Additive Manufacturing Developments
Additive manufacturing (AM) technologies enable a component to be fabricated from a 3-D digital "master" computer file by iteratively building 2-D layers and joining each to the layer below. This enables rapid reconfiguration of designs in CAD software, minimal capital investment and the ability to create complex devices built as a single piece.
Until very recently, AM has predominantly been used for prototyping but recent developments across multiple industries indicate AM is now at an inflection point. Several large companies including Hewlett Packard (HP) and General Electric are heavily involved in the development and application of AM technologies for mass-production.
Medical Applications of AM
AM has seen medical applications predominately in the orthopedic and implant sectors. May 2016 saw the announcement by Johnson & Johnson of a partnership with HP to exploit 3D printing for distributed manufacturing models and patient-specific products. In August 2016, FDA issued draft guidance for additive manufactured devices reflecting increased interest in AM within medical applications.
However, the application of AM to diagnostic applications has received very little attention.
Historical Precedents
Parallels can be drawn between existing diagnostic manufacturing methods and the early semiconductor industry in the late 1940s and early 1950s. Electronic products were expensive and unreliable with components manufactured by a separate process and hand soldered together. The development of the transistor went some way toward addressing the issues but the so-called "tyranny of numbers" issue could not be avoided.
This issue was resolved with the invention of the integrated circuit by Kilby & Noyce in the late 1950s at Texas Instruments. This development enabled the integration and miniaturization of electronic circuits and provides a historical precedent for the resolution of issues similar to those experienced today in diagnostic manufacturing.
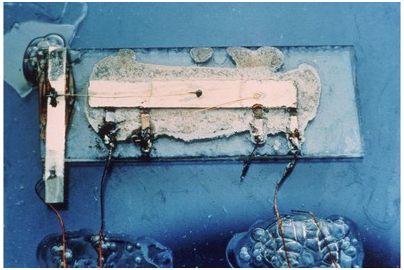
Figure 1: Original Integrated Circuit built by Jack Kilby.
Technical Precedents
Recent developments illustrate the potential of AM-enabled PoC diagnostics. The first of these is the integration of optical and fluidic functionality by Comina et al. This group demonstrated a 3-D-printed disposable device with integrated fluidics and custom optics, coupled to a cell phones camera for illumination and image acquisition. The device enables quantitative glutamate detection with autonomous operation and is therefore a concept suitable for PoC applications.
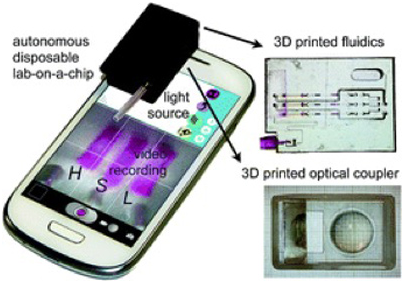
Figure 2: 3D printed, disposable diagnostic device by Comina et al.
Another powerful illustration of the potential of AM enabled PoC instrumentation is a development in an adjacent field by the Wyss Institute for Biologically Inspired Engineering at Harvard University. The Wyss team developed an entirely 3-D-printed system with integrated sensors paving the way for a new class of complex, customizable, multi-material instrumented systems only possible using AM techniques.
The key innovation here is the use of a digital manufacturing procedure enabling an instrumented system to be quickly fabricated with customizable size, shape and other physical properties. This development holds great promise for the fabrication of AM-enabled diagnostic instrumentation with sufficient sensitivity and specificity required of diagnostic systems used outside a traditional lab setting.


Figure 3: Wyss Institute AM fabricated system; fabrication of sensing element (left image) and fabrication of liquid wells above each sensor (right hand image).
These separate developments highlight existing precedents for AM-enabled fluidic, electronic, and optical instrumentation and the potential for a new generation of diagnostic PoC instrumentation.
The Benefits of AM-Enabled PoC Diagnostic Systems
The technical precedents shown above highlight the potential of AM techniques to create a new generation of PoC diagnostic instrumentation that has significant advantages in design and manufacture over existing diagnostic systems.
Design benefits:
· The 3-D design freedom enabled by AM techniques permits close integration of instrumentation domains (fluidics, electronics, etc.) not possible with conventional fabrication methods. This allows the system to be customized around individual test chemistries and also reduces the overall physical size of the system, especially relevant to PoC applications.
· Close integration of different functionality enables new system architectures & partitioning of greater functionality onto the consumable blurring traditional diagnostic system boundaries between "instrument" and "consumable."
Manufacturing benefits:
· A common platform for fabrication of both prototypes and production designs in higher volumes enables a smoother transition from R&D into production, reducing complexity and costs.
· Capital expenditure is reduced as one flexible fabrication platform replaces product-specific tooling and photolithographic masks.
· Integrated systems reduce part count and assembly complexity, leading to lower assembly costs and increased speed of production.
· Benefits of a reduced part count ripple through the supply chain reducing the complexity of manufacturing logistics and quantity of raw material to be stored.
‘Digital’ configurability:
· The "digital" nature of AM enables the ability to design highly functional devices. Using a digital editor allows importing of functional modules from libraries of fluidic, optical, and electrical components and positioning and customizing them in a system designed for a specific application before pushing "print" to have them realized in physical form.
Vision
These design, manufacturing, and "digital" characteristics will enable a new generation of PoC diagnostic systems. Highly integrated diagnostic systems will be printed rather than assembled and devices with active functionality are created as a single object, rather than individually assembled parts. This capability has tremendous potential for customized diagnostic systems rapidly tailored to individual needs and specific PoC applications.
3-D-enabled functions can also be created in new form factors and structures not previously possible. Examples include creating opto-fluidic designs and combining mechanical and optical structures in the same design. Multi-material printing will also enable the integration of instrumentation elements to the structures, adding another layer of functionality and enabling low-cost systems with sufficient diagnostic sensitivity and specificity.
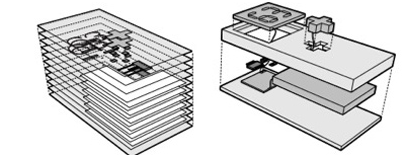
Figure 4: Comparison of layered AM approach to device fabrication (left image) and traditional system construction approach (right image).
Patient & Healthcare System Benefits
A new generation of low-cost, integrated, and miniaturized PoC diagnostic instrumentation would enable new scenarioses within healthcare, providing numerous benefits to patients and the healthcare system.
These PoC diagnostic systems will enable patients to quickly and regularly measure the presence of biomarkers found in small amounts of bodily fluids and capture the data via smart-devices. Transmission of this data will enable consolidation of clinical and diagnostic data held about an individual and cross comparison (using data gathered from multiple individuals) to identify patterns in disease allowing more robust conclusions about diagnosis and treatment to be made.
There are already existing precedents for this smart-device enabled approach. Apple has developed CareKit, a framework for developers to build apps that allow patients to manage their own well-being on a daily basis. A vision of multiple connected devices managed by smart devices has also been proposed as an Internet of Medical Things (IoMT) by the cardiologist and digital medicine innovator Dr Eric Topal.
This approach to healthcare informs the selection of the most appropriate treatment for individual patients (i.e. the right drug at the right time), earlier screening, and smarter monitoring, as proposed by the NHS Personalized Medicine strategy in mid-2015.
The "digital" fabrication benefits of this new generation of PoC diagnostic instrumentation would also address emerging diagnostic scenarioses, allowing rapid configuration of diagnostic systems to enable a more efficient response to disease outbreaks and evolving antimicrobial resistance.
Reasons to Believe
The analogy has already been drawn between limitations of existing manufacturing methods for diagnostics and the early electronics industry in the 1950s.
The invention of the integrated circuit (IC) by Kilby & Noyce at Texas Instruments in the late 1950s integrated large numbers of tiny transistors into a small chip. Increasing levels of integration eventually led the emergence of Moore’s Law, an exponential law that has resulted in the latest commercially available Intel chips with transistors of just 14nm used in powerful notebooks and laptops.


Figure 5: Comparison between the first Integrated Circuit and AM enabled Instrumented system.
AM provides the opportunity for the integration of functionality in multiple domain areas (e.g. microfluidic, optical, electronic, thermal) in a manner analogous to the invention of the integrated circuit. 3-D-enabled AM techniques can create a new generation of integrated, miniaturized PoC diagnostic instrumentation based on existing, proven chemistries.
While there are significant technical hurdles, overcoming these will enable AM to bring the same benefits to diagnostics as the integrated circuit bought to electronics and enable devices such as i) microfluidic-enabled wearables, ii) compact molecular diagnostic chips with sample preparation and integrated detection apparatus, and iii) further simple, affordable, and smaller "plug and play" systems already demonstrated by Oxford Nanopore Technologies & Biocartis.
The last 50 years of integrated circuit development provide the development roadmap for new diagnostic systems with potential to realize significant benefits for patients and the wider healthcare system.
